Contrary to popular belief, early formulation development is not simply dumping API in a vehicle and gavage feeding an animal. If you do this, you will most likely lose time and money with unnecessary and confusing PK studies. This led to the development of our SMART biopharmaceutical solutions that offer input from our scientific advisory board: A one-stop shop from candidate selection through GMP FIH.
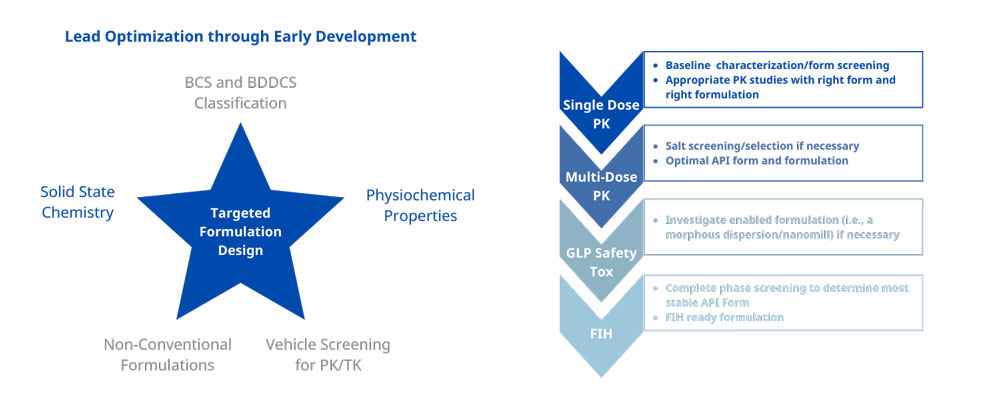
This is an integrated approach to finding the right form and formulation at every step of the development cycle using decision trees and guidance documents built from our over 80 years of innovative pharmaceutical experience. Using our preclinical formulation development services—delivered through our SMART biopharmaceutical solutions—will lead to the shortest possible development timelines with limited resource utilization.
Our formulation approaches to deliver molecules to support PK/Efficacy/Tox studies include tox formulation development, solution and suspension formulation selection through vehicle screening, and solubilization through pH adjustment, co-solvents, surfactants, cyclodextrin, and in-situ salt formation. In addition, we also apply special drug delivery technologies for insoluble compounds.
Conventional - Solution/Suspension Formulations
- pH Adjustment
- Co-solvents
- Surfactants
- Cyclodextrin
- In-situ Salt
Enabling - Enhance Solubility & Bioavailability
- Amorphous Solid Dispersion
■ Spray drying or Lyophilization
- Size Reduction
■ Nano-milling: Nano-suspension
■ Micronization by Jet-milling
- Lipid-based Formulation
- Self-emulsifying Drug Delivery Systems (SEDDS)
|  |  |  |  |
| Micronization 1~10 μm | Nano-milling <1000 nm | Spray Drying ASD | Freeze Drying Lyophilization | Extruder Liposome |
Have a question or need support with your project? Please complete the form, and our team will get back to you shortly.
Our capabilities span three specialized platforms:
Small Molecule
Crystal Bio Solutions
Crystal NAX
By providing your e-mail address, you agree to receive an e-mail response from Crystal Pharmatech to your inquiry. The information you submit will be governed by our Privacy Policy.
Subscribe to be the first to get the updates!